BlueDop Medical is an innovative medical
technology company that has developed a novel
approach to detecting vascular disease,
particularly in the lower limbs. Our flagship
product, BlueDop, offers a more efficient and
accurate alternative to traditional Ankle Brachial
Index (ABPI) screening methods.
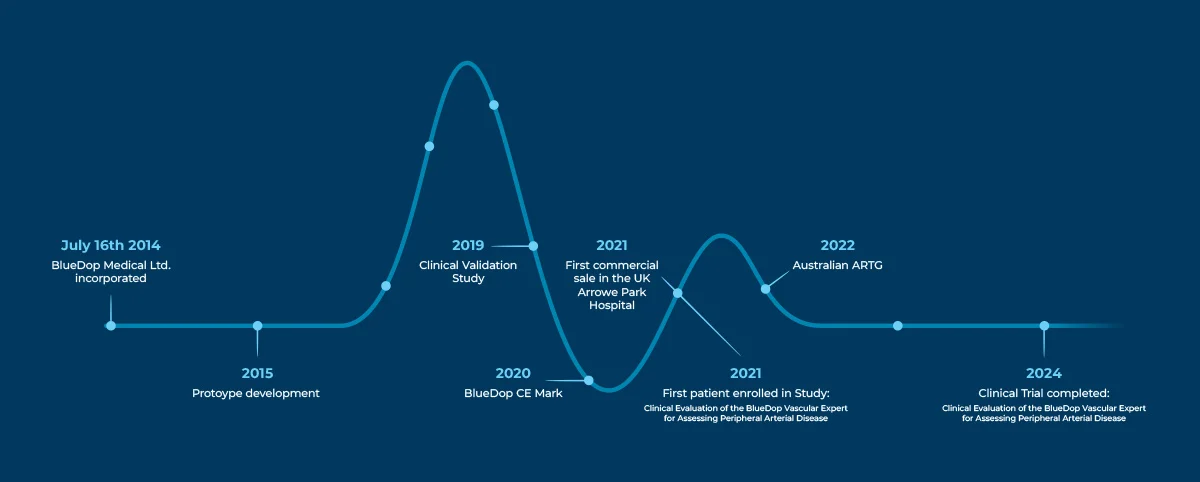
Our Journey
Leadership Team

Declan Keane
INTRIM CHIEF EXECUTIVE OFFICER

Peter Phillips
CHIEF OPERATING OFFICER
Peter Phillips serves as the Chief Operating Officer at BlueDop Medical, where he leverages over 30 years of leadership in the medical device and healthcare industries. Before stepping into his current role, Peter contributed as a Non-Executive Director on BlueDop’s Board, bringing strategic oversight and valuable governance. He continues to expand his influence in the industry as a Non-Executive Director at EXSTENT, underlining his expertise in guiding medical device companies to success.
Peter’s distinguished career includes key leadership positions such as CEO of OxVent and Managing Director at Lombard Medical, where he spearheaded the European launch of pioneering medical products and played a critical role in fundraising and IPOs. His tenure as Chief Technology Officer at Lombard Medical Ltd involved overseeing clinical studies, regulatory compliance, and intellectual property management, showcasing his proficiency in operational strategy and FDA compliance. With a robust background in corporate strategy, commercialization, and board-level leadership, Peter is dedicated to driving operational excellence and strategic growth at BlueDop Medical.

Ross Robinson
VP OF MEDICAL AFFAIRS

Jo Cronin-Wojdat
HEAD OF QUALITY & REGULATORY
Board of Directors
Dr. Patrick Kelly
CO-FOUNDER & INTRIM CHAIRMAN
Konstantin Mikhailov
NON-EXECUTIVE DIRECTOR
Ciaran McCourt
NON-EXECUTIVE DIRECTOR

